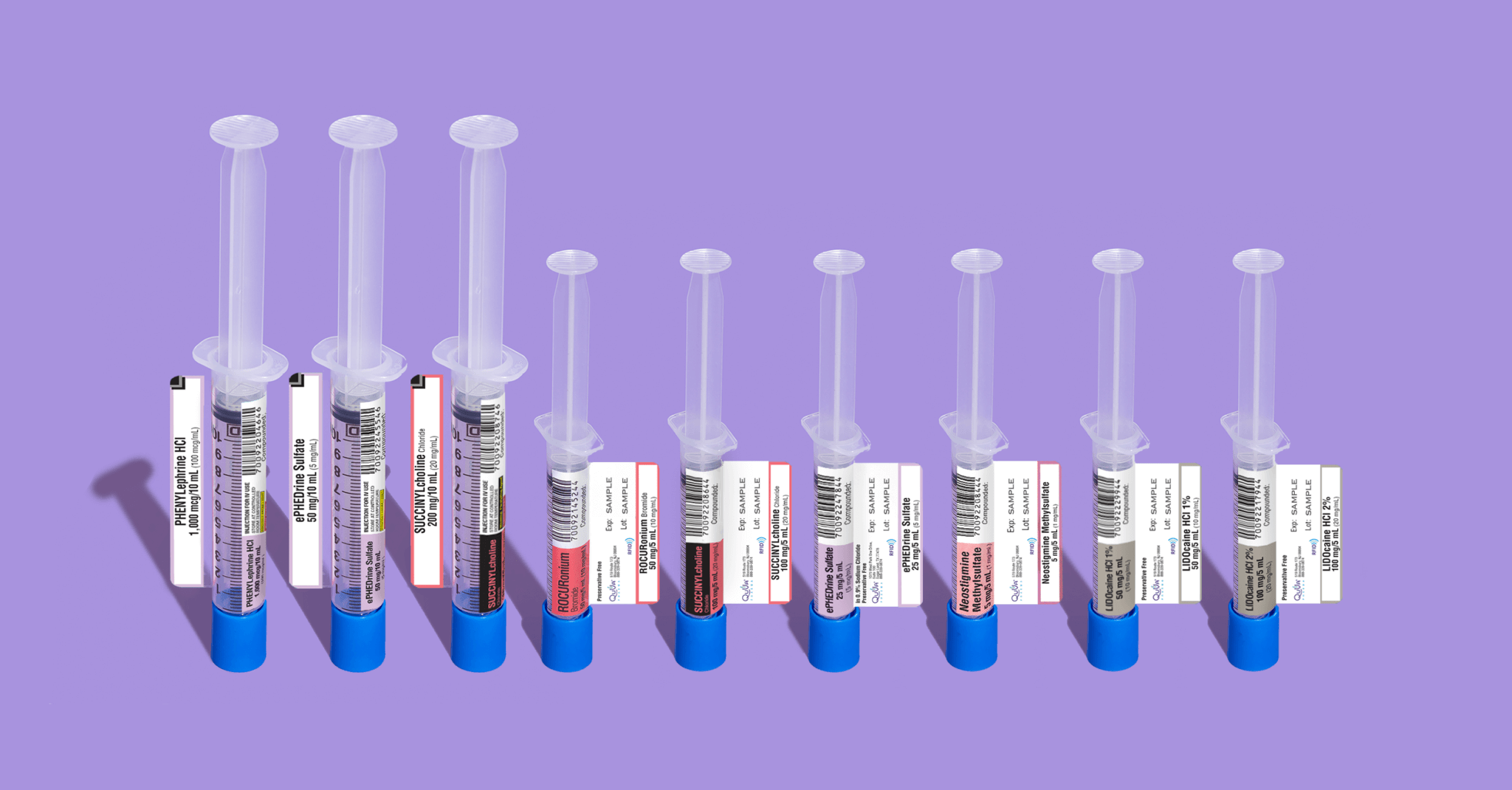
—QuVa increases its portfolio of RFID-enabled, ready-to-administer compounded sterile products (CSPs) to enhance medication management and tracking—
SUGAR LAND, TX – October 28, 2020 – QuVa Pharma, Inc. announces it has expanded its partnership with Kit Check to support its increasing portfolio of products that include embedded RFID tags. The embedded RFID tags allow hospital pharmacists to add batches of medications to inventory quickly and efficiently, and track throughout the hospital—from pharmacy to patient.
“QuVa’s focus is on improving patient care by making 503B compounding more safe, efficient, and reliable. Expanding our portfolio of Kit Check RFID-tagged compounded sterile products will advance quality and safety to improve patient care,” states Stuart Hinchen, CEO & Co-founder, QuVa Pharma. “By tagging our ready-to-administer products, we help Pharmacy improve operational efficiency, inventory management, and reduce risk of medication errors.”
QuVa will also utilize Kit Check’s tunnel association technology and register its products in the cloud-based registry. QuVa’s product labels will be easily identified as RFID-ready and the RFID tag will be fully incorporated in the products’ primary label to enable easy readability and accommodate hospital product storage requirements. Combined with Kit Check’s machine learning-backed diversion detection software platform, this item-level tracking provides pharmacists actionable insights such as real-time restocking, inventory benchmarking, and insight into potential diversion attempts.
QuVa Pharma, a national leader in 503B sterile compounding, is also certified by DoseID — the first-of-its-kind RFID certification body for the pharmaceutical space, of which Kit Check is also a member.
“We are very excited to expand upon our previous partnership with a high-quality, leading 503B partner like QuVa Pharma,” said Kevin MacDonald, Co-founder and CEO at Kit Check. “Pre-tagged medications are a preferred approach for many of our hospital pharmacy clients and embedding Medication Intelligence capabilities into their workflows and processes is becoming increasingly important to keeping a safe and high-quality drug supply.”
For more information, please see QuVa’s Kit Check ready-to-administer syringe portfolio, available for download.
About QuVa Pharma, Inc.
QuVa Pharma is a nationally recognized, industry-leading, cGMP compliant FDA registered 503B services platform and partner of choice for compliance-oriented healthcare facilities looking to ensure a quality, safe, and consistent supply of medications. The company offers a broad portfolio of ready-to-administer products across pain management, anesthesia and OR syringes, anti-infectives, labor and delivery, cardiovascular therapeutic areas, and others. All products are distributed only once sterility and potency testing are successfully completed, and with validation supporting appropriate Beyond Use Dating (BUD). The company is committed to having a patient-safety orientation, as well as a robust product portfolio, leading compliance and safety standards, and being collaborative and transparent in service of their customers.
For product ordering inquiries, please contact QuVa Pharma Customer Service at 888.339.0874 or via email at: Customer.Service@QuVaPharma.com.
For media inquiries, please contact: Mike.Scouvart@QuVaPharma.com.

